Randomized, Placebo-Controlled Trials
- Multicenter Study (120 patients, 1 month):
- IBS Severity: Enterofytol® achieved a significantly greater decrease in the IBS Symptom Severity Score (IBS-SSS) compared to placebo at all time points.
- Pain Relief: 64% greater reduction in abdominal pain over placebo.
- Quality of Life: Marked improvements across multiple quality of life domains (hypochondria, body image, food avoidance, social reaction, and more)1.
Real-World Observational Trials
- 253 patients (2 months):
- Symptom Severity: 45% reduction in total IBS-SSS from baseline.
- Abdominal Pain & Distension: Decreases of 55% and 54% respectively at two months.
- Quality of Life: 33% improvement in overall IBS-QoL score.
- Additional Outcomes: Reduction in frequency and improved consistency of stools, decrease in sick leave, and fewer pain locations reported.
- Tolerability: Excellent safety profile; no significant adverse effects observed1.
Scientific Conclusions
- Enterofytol® was consistently superior to placebo for reducing both core and secondary IBS symptoms.
- Improvements were notable as early as 10 days and maintained for up to 60 days with continued use.
- The unique combination of bio-optimized curcumin and fennel oil addresses both the functional symptoms and underlying inflammatory/metabolic disturbances of IBS.

Multicenter, double blind, randomized placebo-controlled study on 120 patients with Irritable bowel syndrome Treatment duration: 1 month
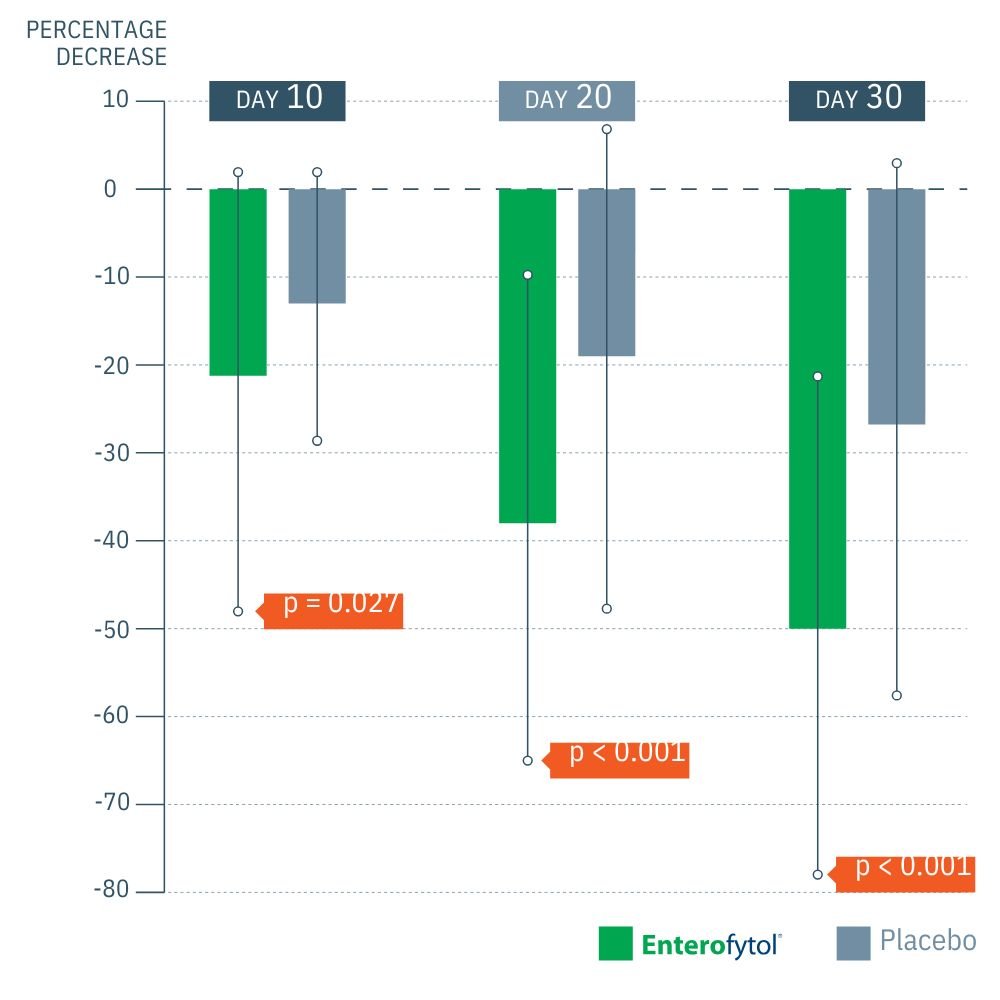
RESULTS
(1) Significantly greater decrease in IBS Symptoms Severity Score. (IBS-SSS)
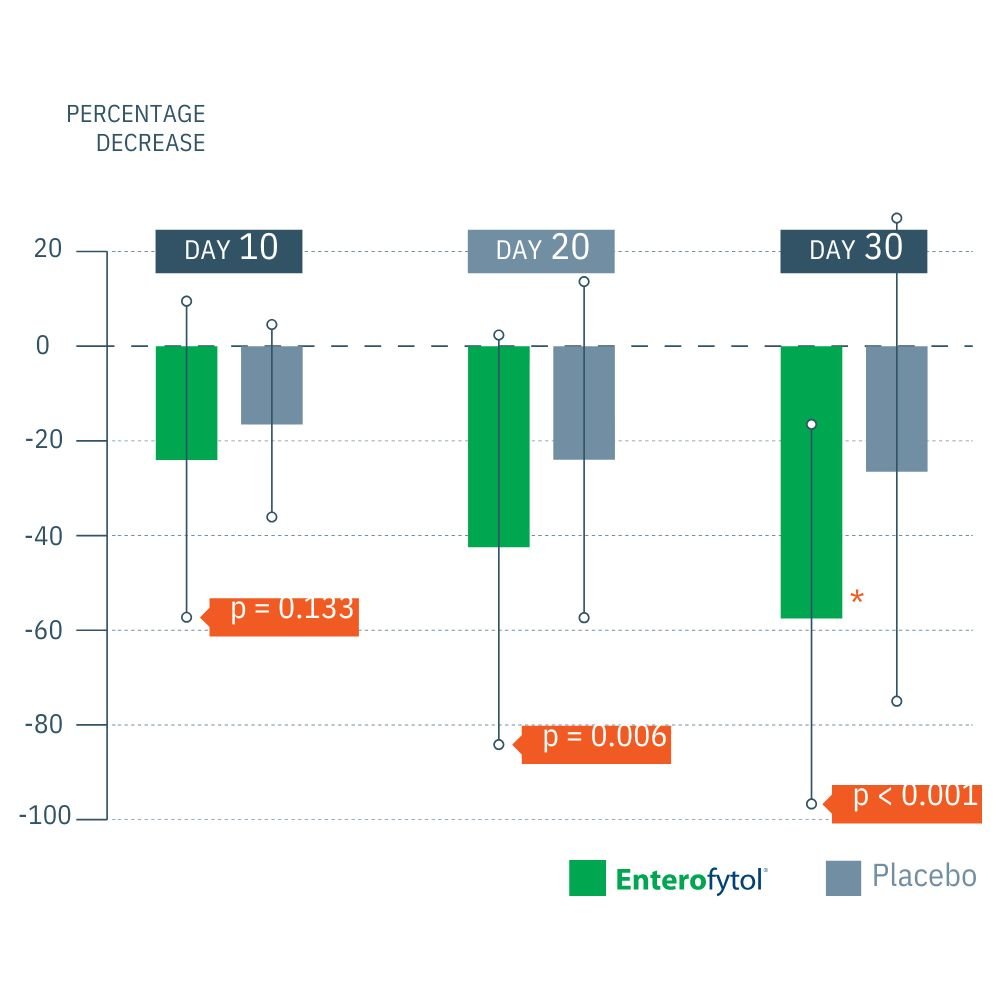
(2) Significantly greater decrease in abdominal pain (visual analogue scale pain)*64% less pain compared to placebo

253 patients (studywith70general practitioners in Belgium) Evaluation of Enterofytol® efficacy in patients suffering from Irritable Bowel Syndrome (IBS)

(1) Significant reduction in total IBS Symptoms Severity Score (IBS-SSS) (p>0.0001)-45% inIBS SSS vs. baseline



